-
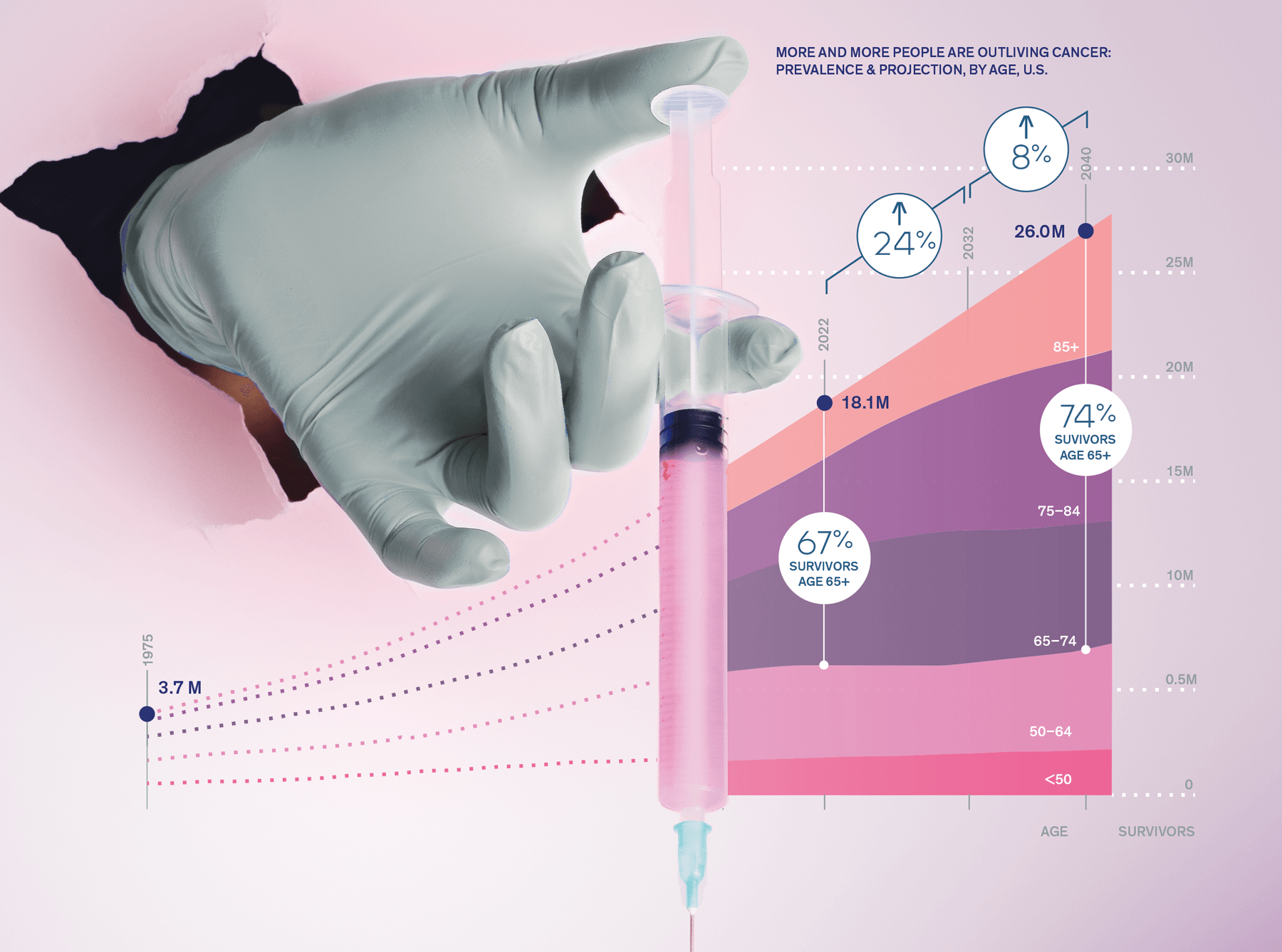
8MIncrease in cancer survivors
by 2040
-
75%Pediatric cancer survival rate increase
since 1950
-
10%Stage 4 cancer survival rate increase, US
2018–2025
In 1950, pediatric leukemia killed 90% of afflicted children. Back then, tobacco had yet to be causally connected with lung cancer, and the only breast cancer treatment available was a radical mastectomy. Chemotherapies were arriving, but were frequently as fatal as the cancers they were trying to treat.
Today cancer remains the second leading cause of death in the US after heart disease, but many cancers are in retreat as a raft of new treatments and discoveries come online. For instance, pediatric cancer survivability has increased from 10% in 1950 to 85% today. Targeted therapies pinpoint cancer-causing mutations and save more patients with fewer side effects. These include drugs that activate a person’s own immune system to attack and neutralize tumors. By 2040, cancer survivors—patients who have been cancer-free for at least five years—will increase from 18 million to over 26 million people in the US.
Globally, nearly 20,000 clinical trials are underway, and spending on cancer drugs is slated to reach $269 billion by 2025. The US FDA is on track to approve an unprecedented number of new cancer treatments in the coming years, continuing a brisk pace that has seen over 80 new cancer drugs approved since 2015— one quarter of all pharmaceuticals approved.
-
25%of all pharmaceuticals approved since 2015 are new cancer drugs
-
20KClinical cancer trials in progress, global
Detecting cancer in its earliest stages is a major focus of President Biden’s 2022 Cancer Moonshot Initiative, which earmarked $200 million in grants issued by the Centers for Disease Control (CDC) for cancer prevention and screening. This includes a massive Multi-Cancer Early Detection (MCED) study backed by the National Cancer Institute (NCI). These “liquid biopsies” hunt for multiple cancers in a single blood sample. A test developed by Grail, a Menlo Park–based cancer detection company, became the first MCED test commercially available in the US in 2021.
Other programs that will help sniff out cancer include the Cancer Moonshot Biobank, which will collect tumor specimens and share samples with researchers to advance care. Also look for expanded use of nanotechnology in imaging, and for AI in biosensors and wearable technology to improve early detection.
Scaling and screening access will remain a challenge in remote and underserved regions of the US and in lower-income countries. The NCI has earmarked $23 million to create Telehealth Research Centers of Excellence to study how telehealth affects cancer care. Global events like pandemics, wars and natural disasters will also continue to disrupt early detection efforts. For instance, 10 million screenings were missed during the Covid-19 pandemic.
The rise of phenomics—the analysis of comprehensive personal biological data ranging from a person’s DNA to metabolites—is also combining with other big data to provide sophisticated profiles of a person’s risk for cancer and other diseases.
One pandemic silver lining: the improvement of remote research processes due to researchers being forced to isolate. Companies like Benchling in San Francisco have developed cloud-based laboratory information management systems that streamline everything from protein modeling to collaborations. Clinical trials also went largely remote, as new treatments were monitored and administered more at home, setting the stage for a future where those formerly without access to hospitals and clinics can receive care. This will benefit isolated and other historically underserved populations.
Data science is disrupting each step of the drug development process.
Yizhen Dong
Healthcare venture investor
Immuno-oncology (IO) aims to rev up a patient’s immune system to recognize and kill cancer cells. IO strategies include monoclonal antibodies, checkpoint inhibitors, cytokines and CAR T–cell therapy. Early results have been extremely promising. The checkpoint inhibitor dostarlimab produced 100% remission in patients with rectal cancer in a small trial published in the New England Journal of Medicine in June 2022. In November, Nature published results from the first human trial in which CRISPR-engineered immune T cells successfully blocked cancer progression, paving the way for improved CAR T–cell therapies using CRISPR technology.
Cancer vaccines are also a promising new cell therapy that can treat cancer by helping the immune system recognize and attack tumor cells. Similar to vaccines for infectious diseases like Covid-19, these vaccines can be made from mRNA-encoding cancer peptides, effectively teaching immune cells what to go after. If a current crop of early-stage trials proves successful, some will be approved within the next decade.
The gut microbiome also plays a role in immune responses, cancer growth and treatment outcomes. Recent CAR T immunotherapy studies showed that use of antibiotics therapy disrupted the gut microbiome and was associated with worse outcomes. Future treatment strategies that enlist the microbiome include highly personalized probiotics, targeted antibiotics, fecal transplantation and targeted microbiota injection into a tumor.
Political leaders will increase their bets on immuno-oncology. The US Patent and Trademark Office is fast-tracking immunotherapy applications, and an immuno-oncology expert, Dr. Elizabeth Jaffee, was appointed as chair of the President’s Cancer Panel.
Precision medicine is not the standard process for treating cancer yet, but I see that door opening.
Andrew Hessel
Microbiologist, geneticist and entrepreneur
-
Less Than 40%Oncologists confident using genetic sequencing technologies, US
What we call cancer is actually a constellation of rare diseases. In the next decade, pharmaceutical companies will be challenged to rethink their standard business model that looks for blockbusters. Developing individualized therapies will become the new holy grail. Healthcare systems will need to become more agile and retrain oncology teams as more targeted therapies are approved, each one for smaller groups of patients.
Currently, only a fraction of cancers are treated with targeted agents. In 2020, nearly a quarter of lung cancer patients in the US did not get genomic testing before first-line treatment despite the availability of multiple targeted treatments based on a patient’s DNA. Fewer than 40% of US oncologists in a survey felt confident using new sequencing technologies.
In addition to targeting cancer mutations, nanotechnology makes drug delivery more precise. Nanoscale antibody-drug conjugates deliver a safer, more potent form of chemotherapy for solid and hematological cancers. Coupling nanotech with radiation and immunotherapy is on the horizon. As physicians increasingly adopt genomic sequencing and targeted approaches in the coming decade, look for a significant increase in cancer survivors.
-
40% HigherMortality from breast cancer for black women vs. white women
While many may think of technology solutions as objective and unbiased, recent clinical studies have demonstrated how these products often amplify the biases of their creators and can further exacerbate health inequities.
Félix Chinea, MD
Head of Diversity, Equity, Inclusion and Belonging at Doximity
At first, accessibility will remain limited. Patients from historically underserved populations will experience delays and find it harder to get individualized treatments. But organizations from the AMA to the White House are making equity and access a key priority.
New companies are targeting overlooked communities, including Boston-based Folx Health, which addresses health concerns of LGBTQ+ patients, who are often stigmatized in traditional clinics. TrialJectory, in New York, matches cancer patients to clinical trials and boosts participation by underrepresented groups. Surveys show receptiveness to medical mobile apps like Survive and Thrive, which was created by Sacramento nonprofit Carrie’s Touch to support black women with breast cancer, who have 40% higher mortality than white women.
Globally, over 90% of cancer research is conducted in high-income countries, and research lags in low-to-middle-income countries. Areas missed by research include cancers caused by occupational carcinogens, tobacco use and khat chewing. Also underfunded in low-income countries: distribution of vaccines for HPV, which causes cervical cancer.
In a first-of-its-kind deal, Novartis has agreed to license its leukemia drug Tasigna to generics manufacturers for distribution to lower-income nations. If we want to ensure equitable access to care, a benefit to all society, more companies should follow suit.
I see major advances in saving lives from cancer in the next 10 years by eliminating cancer health disparities …by ensuring equity in access to quality cancer care to all persons.
Karen M. Freund, MD, MPH
Physicianin- Chief for the Tufts Medical Center Department of Medicine and Professor of Medicine at Tufts University