Hacking our Repair Systems
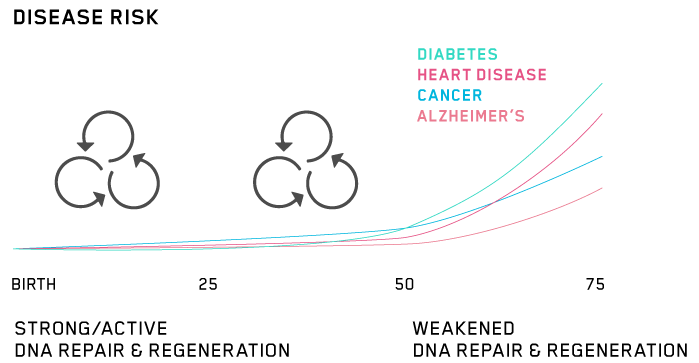
Age is by far the biggest risk factor for heart disease. It’s seven times more important than high cholesterol.
Eric Verdin
CEO of the Buck Institute
Early Inspirations
| A Decade LaterThese scientific investigations into the biochemical pathways of aging have moved incredibly fast from the lab to commercialization. | |
CALORIE RESTRICTIONScientists have recognized since the 1930s that mice on calorie-restricted diets can live 2x longer. It’s shown to work in every species studied. We just didn’t know why it worked or how to safely take advantage of it. Gradually, science has drilled down on the metabolic and cellular mechanisms. A rhesus monkey at the University of Wisconsin is living to the human equivalent of 130 years old on a calorie-restricted diet. | The research on how calorie restriction slows aging has drilled down to the metabolism coenzyme NAD+, which regulates the sirtuin class of cellular mechanism proteins, critical to DNA repair. NAD+ is a coenzyme found in all living cells, but it declines with age. Just putting drops of NAD+ into mice’s drinking water led to obvious age reversal within a week. The muscle tissue of 2-year-old mice soon had qualities identical to that of 3-month-old pups. Trials in humans are in the recruiting stage, and will focus at first on brain repair after a mild concussion. | |
PARABIOSISOld people have just as many stem cells as young people—so why don’t they work just as well? In search of the answer, in 2005, Thomas Rando at Stanford connected two mice together, an old mouse and a young one, to share a circulatory system. Quite simply, the old mouse grew younger—because compounds in the young blood reactivated the stem cells of the old mouse, triggering tissue genesis. A race began to find exactly which compounds in young blood are at work. | When a cell’s chromosomal telomeres shorten to the point it can’t divide anymore, Unity Biotechnology in San Francisco has developed a drug that causes zombie senescent cells to die. In lab tests of their drug, human knee stem cells regain their ability to regrow cartilage. Clinical trials have begun. Meanwhile, the compound rapamycin is already FDA approved. It’s used to temporarily suppress the immune system after organ transplants, but it also blocks senescent cell signaling. By loading nanoparticles of rapamycin into molecules that bind only to senescent cells, we can silence them—without suppressing immune cells. | |
TELOMERASETelomeres are the caps on the end of our chromosomes. Every time a cell divides, the cap shortens. After 50 to 70 divisions, the cell will die because there’s no telomere left. However, the enzyme telomerase works to replace and repair the caps, allowing the cell to divide without limit. For their work on the discovery of telomerase, Elizabeth Blackburn and Carol Greider were awarded the Nobel Prize in 2009. | Several studies involving mouse-to-mouse blood transfusions have demonstrated confirmed antiaging effects. To make the jump to humans, researchers started by injecting mice with young human plasma (from teenagers or from umbilical cords of newborns). The infusion doesn’t just boost the recipients physically, it also improves their cognition and memory. This brain boosting has been connected to the TIMP2 protein present in plasma. Now human-to-human plasma transfusions have begun. A trial is under way at a hospital in South Korea to test whether injections of human umbilical cord plasma have antiaging effects in older healthy people. The Silicon Valley startup Alkahest has found no safety concerns using teen blood plasma in Alzheimer’s patients, and is now looking at its efficacy. Gene therapy that targets telomerase is yet a fourth approach. Essentially it gives the renewal power of stem cells to other cells. While not yet tested in humans, it’s had a remarkable effect on aging mice, delaying or reversing osteoporosis and insulin sensitivity, and improving muscle strength and coordination. This approach had been considered dangerous because, presumably, it might make cancer cells immortal, too. However, scientists have successfully found a way for this gene therapy only to target slowly dividing cells, opening the door to a permanent solution to prevent cellular senescence. |
Sperm and eggs from older people still have the power to create a brand new baby. The genetic capacity for youth is in us all. Learning from that, we can trick our cells into thinking they’re younger than they are.
Elizabeth Blackburn
Nobel Prize winner
